
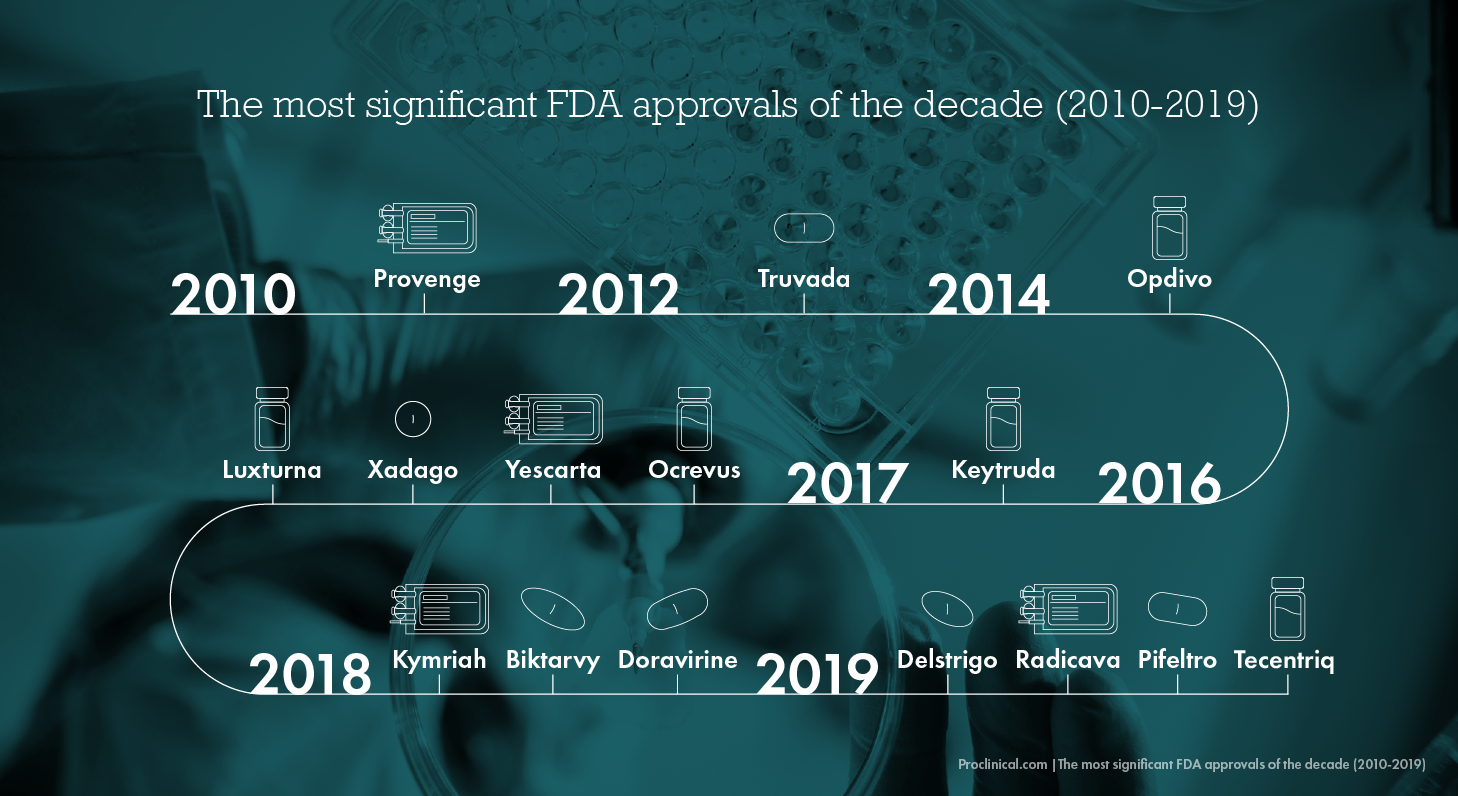
From innovative leaps in cancer diagnosis and treatment to the growing force of artificial intelligence in aiding medical discovery, the 2010s was a ground-breaking decade for the life science industry. We have seen the steady rise of targeted and personalised medicines, and advancements in genetic engineering have thrown up controversial yet undeniably ground-breaking potential that could completely change the way we diagnose and treat disease in the future.
To bring these incredible drug breakthroughs to US patients, the Food & Drug Association (FDA) approved a significant number of innovative drugs and therapies between 2010-2019. 2017 was a record-breaking year – with the FDA approving 55 drugs, and in 2019, there was still an impressive 48 innovative medicines approved.
Many of these medicines were approved by other industry bodies elsewhere in the world, such as the European Medicines Agency (EMA) and China’s National Medical Products Administration (NMPA) increasing the accessibility of these new, life saving treatments to patients globally.
The last decade has been truly outstanding for medical innovation and discovery. Below are some of the most significant FDA approvals of the 2010s, within the context of the top 5 biggest areas of medical advancement.
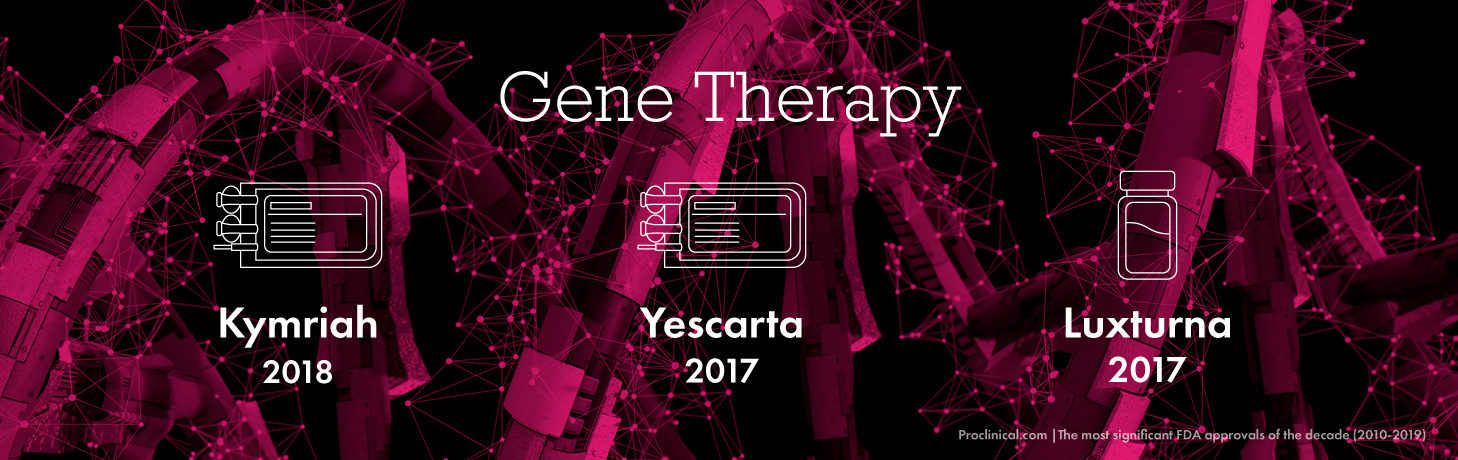
Gene therapy
Gene therapy is a type of treatment that works by replacing damaged, defective or missing genes that cause disease in a patient, with normal healthy genes. Latest advancements in this area include the highly controversial CRISPR technology, which could give us the power to fully manipulate a person’s DNA to eradicate disease.
The FDA have made a number of gene therapy approvals over the last decade, bringing hope to patients suffering with a variety of very serious conditions like cancer and genetic disease. Gene therapy treatments are an excellent example of the move towards personalised medicine, as these drugs are individually tailored to each patient. Some of the most important gene therapies approved by the FDA this decade include Kymriah (developed by Novartis in 2018) and Yescarta (developed by Kite Pharma in 2017) which both treat different types of lymphoma, as well as Luxturna in 2017 – Spark Therapeutics’ therapy to treat an inherited eye disease called IRD which caused blindness.
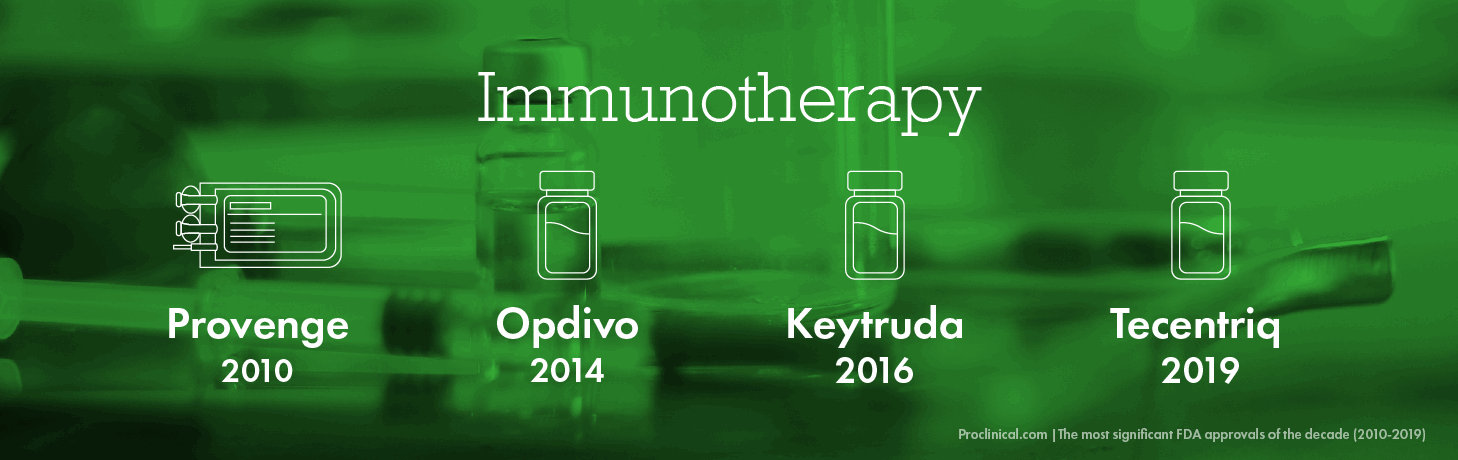
Immunotherapy
The concept of immunotherapy is not new, dating back to the late 1800s when physicians attempted to stimulate a person’s natural immune system to fight disease. However, in the last ten years, immunotherapy has transformed into a complex technology with untold potential to treat a range of life-threatening diseases. It has been particularly ground-breaking in cancer, completely changing the landscape of how to treat the disease for years to come. The technology is also helping researchers understand more about the mechanics of the disease, which may lead to an eventual cancer cure.
The first break-through immunotherapy approved by the FDA was Provenge in 2010, a cancer vaccine that was proven to effectively treat multiple cancers, from melanoma and lymphoma to lung and liver cancers. As the technology become more advanced, various other FDA approvals followed, including Opdivo in 2014, Keytruda for multiple types of cancer in 2016 and Tecentriq for lung cancer in 2019.

Rare Diseases
Rare diseases, also referred to as orphan diseases, are a group of around 7,000 known diseases that affect a small percentage of the population, usually 1 in 200,000. However, in the USA, rare disease is not so rare when grouped together – affecting as many as 1 in 10 Americans. Therefore, the steady increase in FDA approvals for rare disease drugs over the last decade has been a significant achievement.
Most notably, the emergence of RNA interference technology, has been a real breakthrough in the area of rare disease. It works by providing new DNA to cells which modifies them to treat the disease. The FDA approved the first-of-its kind RNAi therapy – Onpattro – in 2018 to treat peripherial nerve disease which is caused by a rare disease called hereditary transthyretin-mediated amyloidosis (hATTR). This promising treatment could be used in future to treat other devastating rare diseases such as Huntington’s disease.
Outside of RNAi, the FDA has approved some other milestone drugs to treat rare disease. These include Crysvita in 2018, to treat a genetic bone disease called X-linked hypophosphatemia (XLH), and Galafold which helps patients suffering from Fabry disease, a rare disease that affects the kidneys, heart and nervous system.
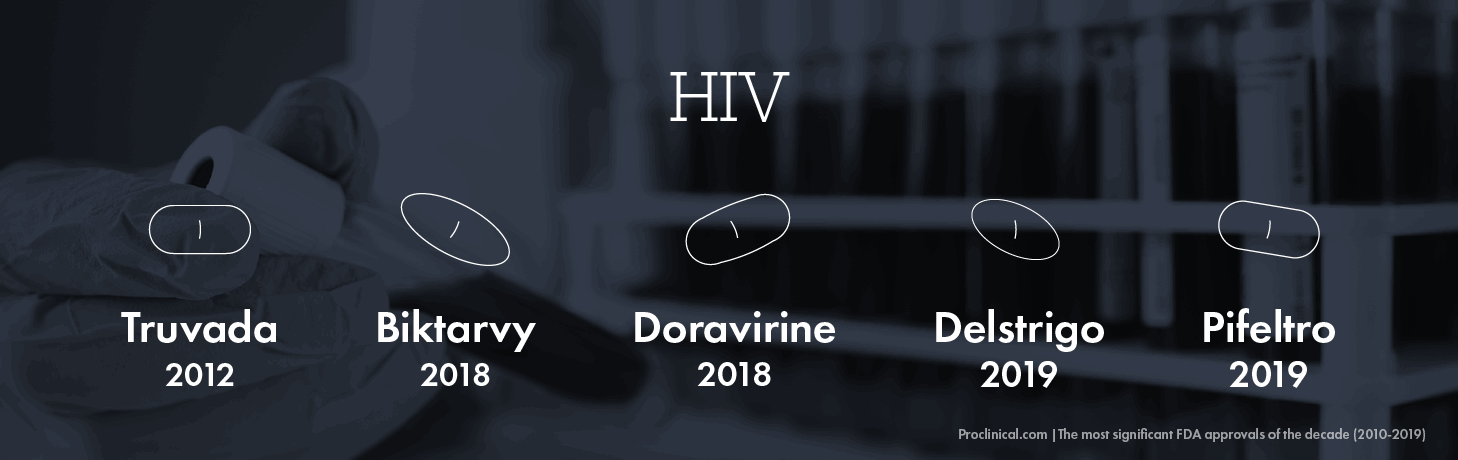
HIV
At the end of the 2000s, there had certainly been significant improvements in the treatment of HIV, with the life expectancy of patients increasing from 2-3 years in the 1990s to several decades by 2010. In the years since then, HIV treatment has made even bigger strides in prolonging and improving the lives of HIV patients globally.
FDA approvals for the treatment of HIV have accelerated in the last five years in particular, with a ground-breaking 9 drugs approved in 2018 alone – out of the total 29 drugs approved since the early 1980s. Out of these many approvals this decade, several stand out.
In 2018, the FDA approved the first single-tablet treatment for HIV – Gilead’s Biktarvy, which proved much more manageable than the standard cocktail of drugs patients would take every day. Merck’s Doravirine, also approved in 2018, is a highly effective combination therapy that showed higher tolerance in patients than older HIV drugs. In 2019, the FDA approved another two HIV drugs by Merck – Delstrigo and Pifeltro that are thought to be the most effective three-drug, single-tablet regimen available to HIV patients who have not had any previous treatment. Although originally approved in 2004 as a treatment for people who are already infected, the approval of Gilead's Truvada in 2012 as a prophylactic carried much greater significance since it can now be used by individuals at high risk as a precautionary measure to stop them from contracting the virus.
Although there is still no cure for HIV, the ability to extend patients’ lives by several decades and enable them to live with fewer and fewer side effects of both the disease and its medication is proving to be one of the greatest accomplishments of the decade.

Neurology
Neurology is one of the most complex areas of science, with researchers still having very limited understanding of how the human brain works, least of all the mechanics the many diseases that affect it. However, in the last decade, the life science industry has made important steps in understanding and treating some of the most devastating neurological diseases that affect millions globally. These include Alzheimer’s, Parkinson’s, multiple sclerosis and motor neuron disease (MND) also known as ALS.
While there have certainly been fewer quick fixes or cures in this elusive field, the mere ability to alleviate symptoms and slow down the progression of neurodegenerative disease has been ground-breaking. The most significant FDA approvals for neurological disease this decade include Xadago (2017) to treats Parkinson’s patients who have stopped responding to Levodopa, the leading treatment since the 1960s.
Multiple sclerosis (MS), a common neurodegenerative disease, had it’s first FDA approval for the primary progressive strain of the disease in 2017 with Ocrevus, which also improved the symptoms of other MS patients. Perhaps among the most devastating of neurodegenerative diseases – MND/ALS – had a real break-through moment in 2017 was the development of Radicava, the only approved drug available to patients today. The long-awaited FDA approval of Radicava gave patients the chance to slow the progression of the disease and improve their ability to carry out day-to-day tasks such as eating and speaking.
As we enter yet another decade, we can look forward to the untold potential of exciting medical technologies such as artificial intelligence, genetic engineering and immunotherapy, as well as continuing to see advancements in growing therapeutics areas such as neurology, antibiotic resistance and HIV.
.png)

.png)


.png)
.png)




.png)
.png)












