
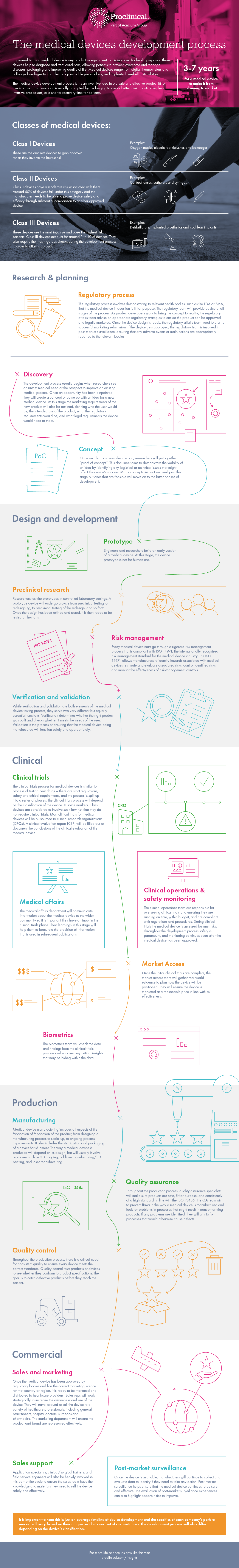
The medical device development process turns an inventive idea into a safe and effective product fit for medical use. These devices help to diagnose and treat patients, allowing patients to prevent, overcome and manage diseases or conditions, prolonging and improving quality of life. Medical devices range from digital thermometers and adhesive bandages to complex programmable pacemakers, and implanted cerebellar stimulators. They are divided into three classes and are ranked based on the level of risk they carry.
The development process usually begins when researchers see an unmet medical need or the prospect to improve an existing medical process. Once an idea has been decided on, researchers will put together “proof of concept”.
After the concept has been approved, the device will move into the design and development phase. At this early stage, engineers and researchers build an initial version of a medical device. A prototype device will then undergo a cycle from preclinical testing to redesigning to preclinical testing of the redesign, and so forth. Every medical device must go through a rigorous risk management process that is compliant with ISO 14971, the internationally recognised risk management standard for the medical device industry. The quality assurance team will also check the verification and validation of the product.
The development process for medical devices is similar to the process of testing new drugs – there are strict regulatory, safety, and ethical requirements, and the clinical trials process is split up into a series of phases. The clinical trials process will depend on the classification of the device. The clinical operations team are responsible for overseeing clinical trials and ensuring they are running on time, within budget and are compliant with regulations and procedures. The medical affairs department will communicate information about the medical device to the wider community, so it is important they also have an input in the clinical trials phase.
Once the initial clinical research is complete, the market access team will gather real world evidence to plan how the device will be positioned. The biometrics team will check the data and findings from the clinical trials process and uncover any critical insights that may be hiding within the data.
After the clinical trials have been carried out, the manufacturing process can begin. Medical device manufacturing includes all aspects of the fabrication of a medical device, from designing a manufacturing process to scale-up to ongoing process improvements. Throughout the production process, quality assurance specialists will make sure products are safe, fit for purpose and consistently of a high standard, in line with the ISO 13485. Quality control tests products of devices to see whether they conform to product specifications.
Once the medical device has been approved by regulatory bodies and has the correct marketing licence for that country or region, it is ready to be marketed and distributed to healthcare providers.
Sales reps, supported by the sales support team, will work strategically to increase the awareness and use of the device. When the device is readily available, medical device companies will continue to collect and evaluate data to identify if they need to take any action. Post-market surveillance helps ensure that the medical device continues to be safe and effective.
See our infographic to discover more on what it takes to get a medical device brought to market:
.png)

.png)


.png)
.png)




.png)
.png)











